-
Mycobacterial ribosomes

Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb). In 2015, TB led to ~1.4 million deaths worldwide. The success of Mtb as a pathogen is largely attributable to its ability to persist in the host and to develop resistance to antibiotics. The Mtb ribosome is a major target for treating TB. It possesses several features that are missing in other ribosomes, including unique ribosomal RNA expansion segments, ribosomal proteins and ribosomal factors, whose structures and functions are not clear. Species-specific ribosomal features may confer increased functionality to the ribosome, in order for the cells to maintain optimal growth or to survive under stress. Understanding the roles of these features will advance our knowledge on the unique physiology of Mtb, particularly its dormancy under stress conditions, which leads to persistent TB infections. In addition, these unique features may provide alternative targets on the ribosome for therapeutics to which Mtb has not developed resistance. Moreover, targeting such Mtb-specific features will not affect the translation in the host, minimizing the side effects of the potential drugs. The primary goals of this project are to study the mechanism of how the novel structural features of Mtb ribosomes impact protein synthesis and to provide structural information that facilitates the development of Mtb-specific therapeutics. This project is supported by the NIH grant P01AI095208 and the Welch Foundation grant A1863.
-
Single-stranded RNA bacteriophages

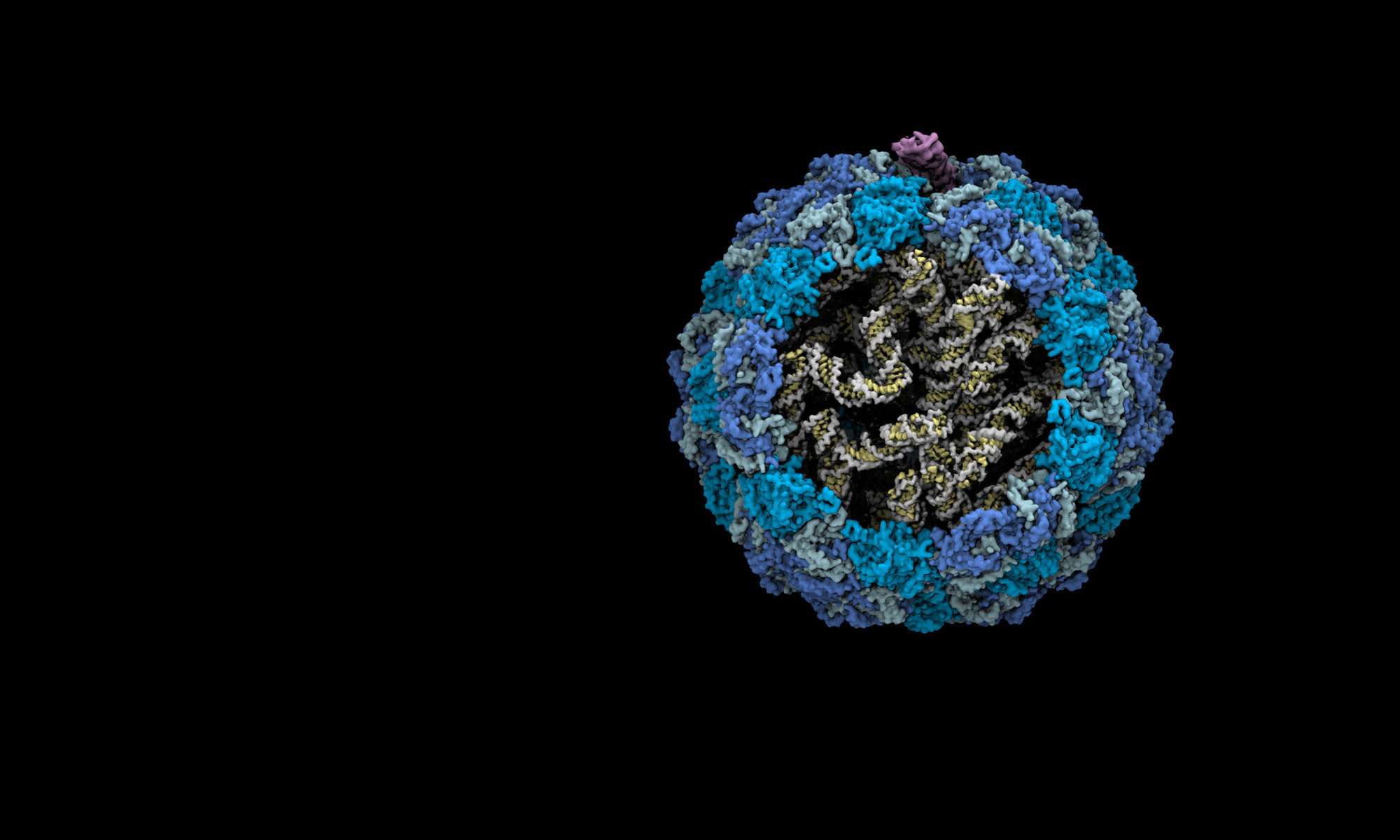
The single‐stranded ribonucleic acid (ssRNA) phages are small viruses which infect their host via retractile pili. This project focus on the structural mechanisms by which ssRNA phages assemble themselves to recognize and destroy their host cells. This is important for a number of reasons: (a) understanding how ssRNA phages recognize and kill bacteria could lead to more specific and potent antibiotics; (b) understanding how ssRNA phages assemble could expand its application for packaging and delivery of other engineered RNA molecules with therapeutic  and biotechnological interests; (c) many of the fundamental processes involved are mirrored in human RNA viruses and in important processes in human cell biology; (d) More ssRNA phages are being discovered and investigated as potential antibacterial agents, so understanding the fundamentals of the ssRNA phage infection cycle is critical. This project is supported by the NSF grant 1902392, NIH grants 1R21AI156846 and 1R01GM141659.
and biotechnological interests; (c) many of the fundamental processes involved are mirrored in human RNA viruses and in important processes in human cell biology; (d) More ssRNA phages are being discovered and investigated as potential antibacterial agents, so understanding the fundamentals of the ssRNA phage infection cycle is critical. This project is supported by the NSF grant 1902392, NIH grants 1R21AI156846 and 1R01GM141659.
-
Clostridium difficile toxins

C. difficile infection (CDI) is the leading cause of hospital-acquired infectious diarrhea and colitis, claiming the lives of ~30,000 Americans each year. The primary cause of symptoms from CDI is due to its secreted toxins, which affect the host cells in the colon. The goal of the current project is to establish a structural framework to reveal how the C. difficile toxins intoxicate the host cell and can be neutralized by engineered non-antibody protein therapeutics, which will provide a novel approach to treat CDI diseases. This project was supported by the NIH grant R21AI137696.
The Zhang lab is also part of the high-resolution cryo-EM data collection consortium supported by the NIH grant U24GM116787.